Protocol
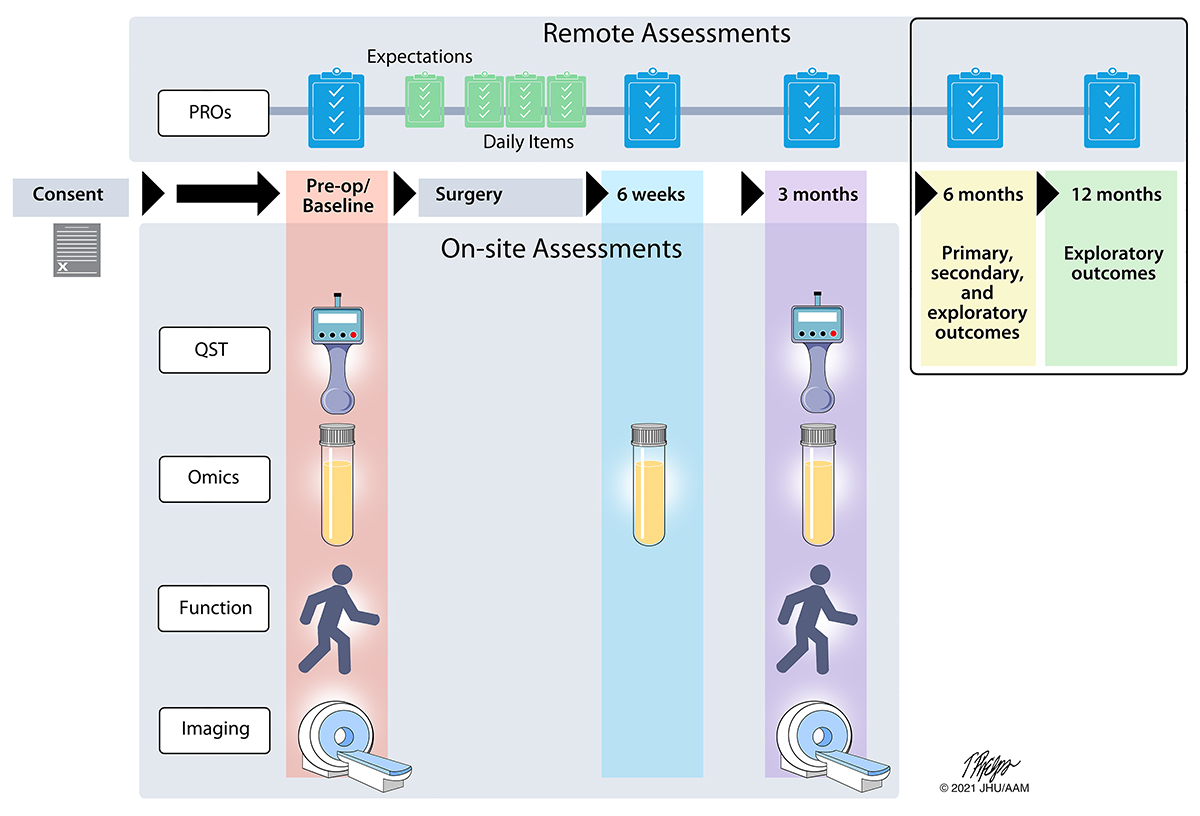
A major aim of the A2CPS study is to identify biomarkers and signatures that can eventually be used to help predict who will or won’t develop chronic pain after surgery. To do that, we’re following nearly 3,000 participants longitudinally - from just before they have surgery until up to 12 months afterwards. Patients who are scheduled for either total knee replacement or thoracic surgery are screened and recruited from two multisite clinical centers (MCCs), one with sites across the state of Michigan and the other in the Chicago metropolitan area. Participants will be asked to complete a comprehensive set of questionnaires remotely prior to and up to 12 months following their surgery. These questionnaires will assess aspects of pain, medication use, disability and function, fatigue, sleep, activity levels, depression, anxiety, and social support. Participants will also attend three clinical visits, one prior to surgery and two following surgery, that will include several clinical assessments. Clinical assessments will consist of pain sensitivity testing (called quantitative sensory testing, or QST), blood draws (for omics studies), functional assessments, and brain imaging (MRI).
For additional details, please read the Protocol paper: Multi-Site Observational Study to Assess Biomarkers for Susceptibility or Resilience to Chronic Pain: The Acute to Chronic Pain Signatures (A2CPS) Study Protocol